
Novel Embolic Device Used for Arterial and Venous Tumor Treatments
February 5, 2021
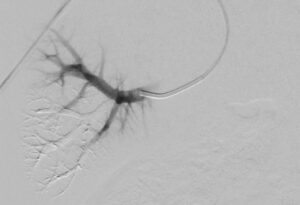
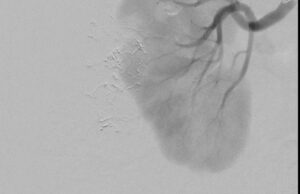
Fluidx Medical Technology has announced successful patient use of the GPX Embolic Device to therapeutically devascularize, or block blood supply to, a tumor.
The GPX Embolic Device is an innovative embolic designed to combine the benefits of other embolics like coils, particles, and liquids with simplified preparation, delivery, precision, and control leading to durable, long-term occlusions.
“This is a significant milestone for the Company,” said Libble Ginster, CEO of Fluidx Medical Technology, Salt Lake City, Utah. “GPX is an advanced embolic technology that overcomes the challenges associated with other embolic devices. GPX can be used effectively for distal penetration and occlusion of vessel networks, as well as proximal “one-and-done” use in conjunction with coiling. We look forward to GPX’s use to help a variety of peripheral and neurovascular patients.”
“Fluidx’s impressive innovative technology offers improvements over current embolic devices, which is a life-changing development for patients,” said Kelvyn Cullimore, president and CEO of BioUtah.
GPX is a low viscosity, aqueous-based solution in a syringe that solidifies into a durable embolic material upon delivery, without polymerization or dimethyl-sulfoxide (DMSO) precipitation associated with other embolics.
Therapeutic, super-selective embolization is a high-growth procedure used for minimally invasive, targeted treatment of internal bleeds, tumors, aneurysms, vascular malformations, uterine fibroids, varicose veins, and other uses.
“We are impressed with the GPX embolic,” said Andrew Holden, M.D., MBChB, FRANZCR, EBIR, ONZM, Director of Interventional Radiology, Auckland City Hospital, Auckland, New Zealand. “We delivered GPX through a long 150cm 2.4F microcatheter from the patient’s radial artery. GPX was easy to use, precise, and occluded the tumor well. We look forward to using GPX across a broad range of applications to improve patient care.”
“GPX is easy to prepare, deliver, and control,” said Ryan O’Hara, M.D., Interventional Oncologist, University of Utah, Salt Lake City, Utah USA. “GPX is responsive to the physician and layers well in the vessel during delivery resulting in improved targeting and control. I see potential for this embolic in interventional oncology and other peripheral vascular and neurovascular uses.”
GPX is packaged in a ready-to-use syringe, requires less than 1 minute of preparation, and may be delivered through standard microcatheters.
Fluidx Medical Technology is a Salt Lake City, Utah based company focused on developing the GPX Embolic Device and other innovative technologies. The GPX Embolic Device is not available for sale in the United States or other markets, and is for investigational use only. www.FluidxMedical.com.
Recent News
- Co-Diagnostics, Inc. Signs Strategic MOU with Partner in Kingdom of Saudi Arabia to Introduce Co-Dx™ PCR Platform to Middle East
- Renalytix Announces Collaboration with Tempus to Advance Intelligent Risk Assessment in Diabetic Kidney Disease
- KUDA THERAPEUTICS AWARDED $5.6M U.S. DoD GRANT TO BRING LEAD ONCOLOGY CANDIDATE INTO CLINIC
- ARUP Achieves Reaccreditation Under College of American Pathologists ISO 15189 Program
- Altesa BioSciences and bioMérieux Announce Collaboration for Altesa’s Upcoming Phase 2B COPD Clinical Trial
- Owlet Makes Premium Monitoring Possible for Every Family With New Dream Sight™ Video Monitor











