
The voice of utah’s life sciences community
As the association representing Utah’s burgeoning life sciences industry, BioUtah strives to develop an ecosystem in which Utah life sciences companies can succeed in developing and delivering technologies that save lives.
Medical Device
Pharmaceuticals
Diagnostics / Laboratory
Biotechnology
Icons made by Freepik from www.flaticon.com is licensed by CC 3.0 BY
Utah Life Sciences by the Numbers
Kem C. Gardner Report Findings Nov. 2023
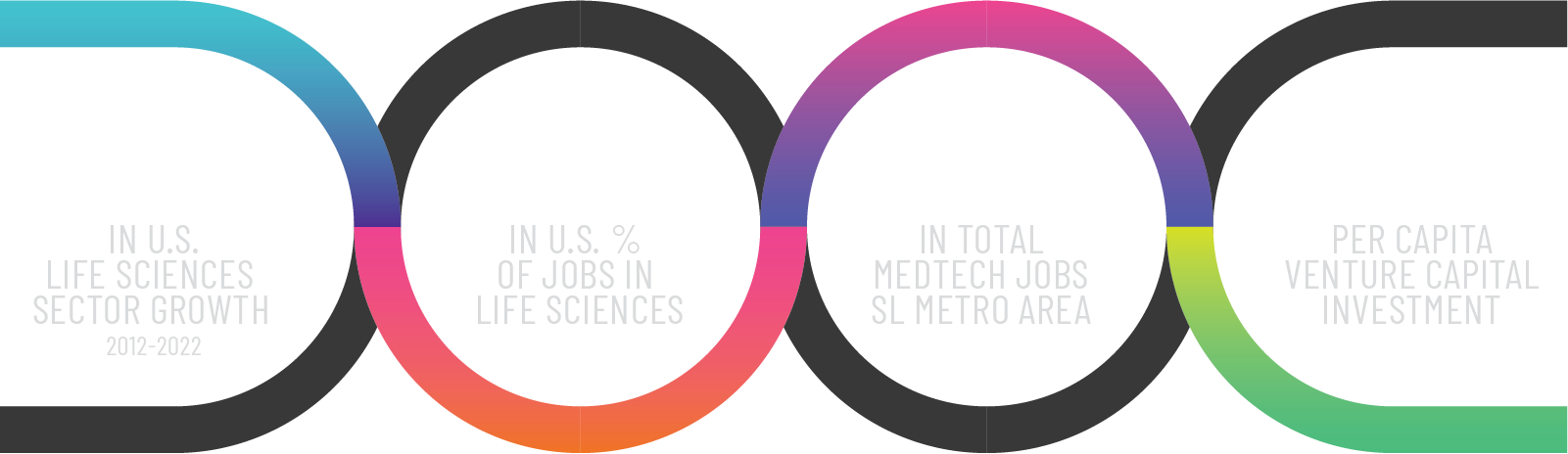
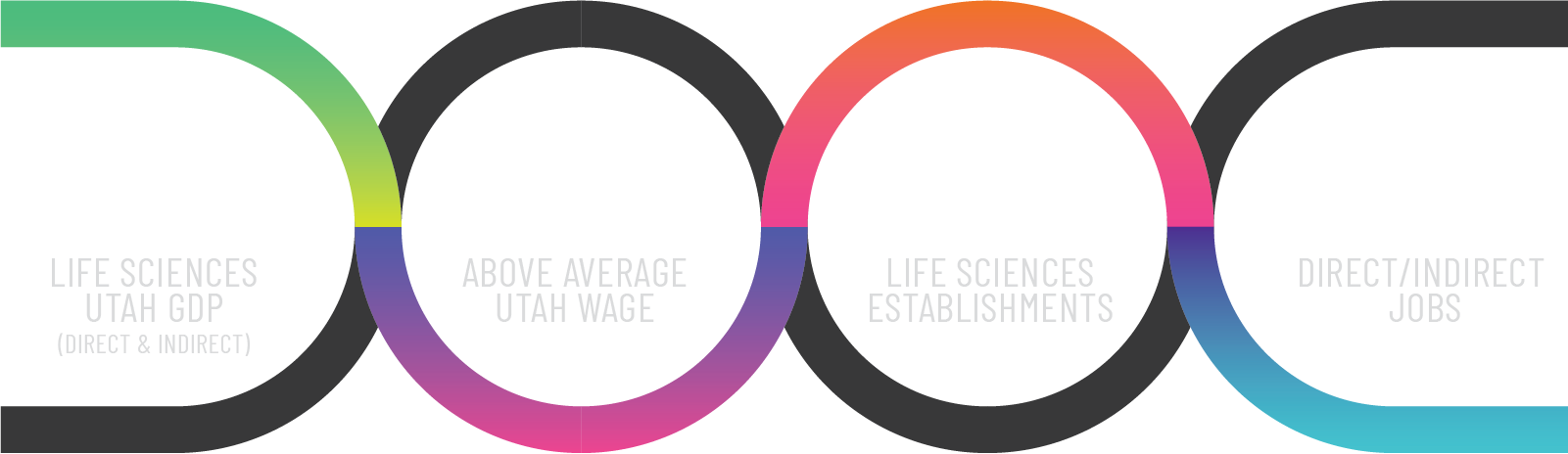
Source: Pace, L. and Brandley, A. (2023), Economic Impacts of Utah’s Life Sciences and Health Care Innovation Industry: Utah’s Life Sciences and Health Care Innovation Industry Creates Substantial Economic Impacts Across the State, Kem C. Gardner Policy Institute, University of Utah.
BioUtah News
Improving Women’s Health: MobileODT Joins Liger Medical
Utah Investment Firm Expands to Deliver Flexible Capital to Transformative Healthcare Companies
Accelerating Development of Therapeutics to Bolster Global Protein Supply Chains
Nestmedic Selects Curavit Clinical Research as U.S. CRO Partner for Pioneering Prenatal Monitoring Trial
Microvascular Therapeutics Celebrates Major Achievements at ASE 2025 Scientific Sessions
BioSeeker™ Powers Development of a First-of-Its-Kind Programmable Therapeutic for Respiratory Syncytial Virus (RSV)
Canary Speech announces it has obtained HITRUST e1 Certification, reinforcing its commitment to cybersecurity related to its voice biomarker technology.
Recursion Acquires Full Rights to REV102, a Potential First-in-Class Oral ENPP1 Inhibitor for Hypophosphatasia
From Here to There
See how BioUtah, the only trade association dedicated solely to Utah’s Life Sciences industry, can help your business grow.

2024 Utah Life Sciences Award Recipients
Lifetime Achievement
Dr. Wm. Dean Wallace
Innovation Impact
Blackrock Neurotech
Entrepreneur of the Year
Jay Muse
Executive of the Year
Bradford Brown
Friend of Industry
Vic Hockett























































