
New Liquid Embolic Multi-Center Trial Successfully Enrolls Final Patient
September 1, 2022
New Liquid Embolic Multi-Center Trial Successfully Enrolls Final Patient
GPX® Trial Completes Enrollment Demonstrating Successful Tumor Uses and Other Applications
Fluidx Medical announces completion of trial enrollment for its novel embolic device, GPX. In this multi-center trial, GPX was used to treat a variety of primary and metastatic tumors, renal adenoma tumors, and a range of other arterial and venous applications.
“We are pleased to announce the enrollment of the final patient in the trial and look forward to participating in future trials using this promising technology,” stated the trial’s principal investigator, Dr. Andrew Holden, M.D., MBChB, FRANZCR, EBIR, ONZM, Director of Interventional Radiology, Auckland City Hospital, Auckland, New Zealand. “In the trial, GPX showed significant potential to advance liquid embolics, penetrating very distally, providing profound embolization, and demonstrating excellent radiopacity which helped to avoid non-target embolization and preserve healthy adjacent tissue.”
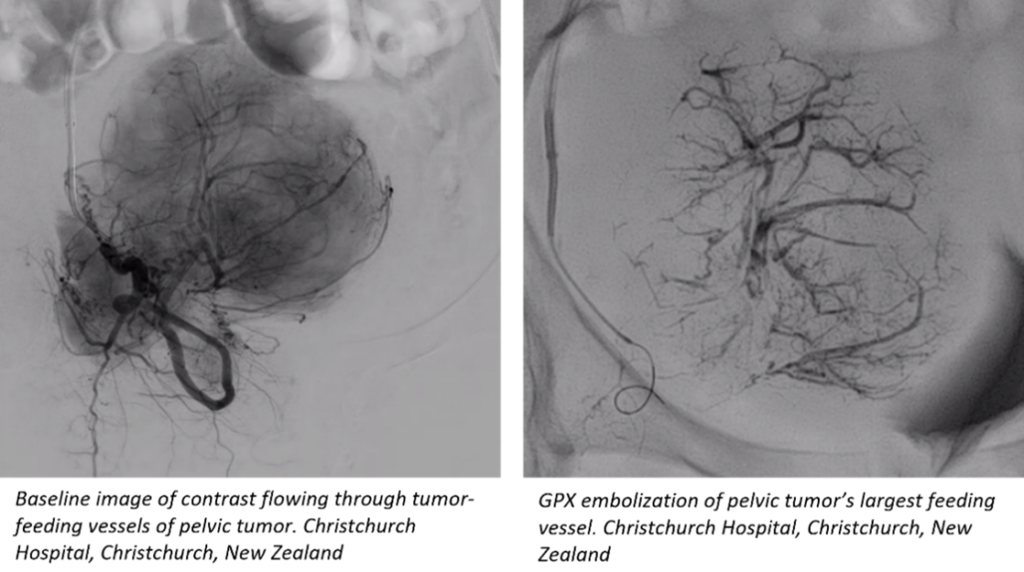
GPX has shown promising results for tumor embolization and other uses where there is a desire for distal vessel bed penetration. Embolization is a procedure in which arterial or venous blood supply to an organ, malformation, aneurysm, bleed, tumor, and/or other abnormal area of issue is blocked. Interim results of the GPX study have been presented at recent annual congresses including Global Embolization Symposium & Technologies (GEST), Society of Interventional Radiology (SIR), and Leipzig Interventional Course (LINC).
Dr. Holden concluded, “We’ve only touched on some of the applications. Further trials will provide opportunities to look at broader applications of this product. We believe it will be an exciting addition to the embolic portfolio for interventionalists.”
GPX® is an innovative embolic designed for simple preparation and controllable material delivery. The device is packaged ready-to-use in a syringe, can be prepped tableside by the clinician in about 30 seconds, and may be delivered through standard microcatheters (no complex mixing systems or special delivery catheters are necessary). GPX technology is a low viscosity, aqueous-based solution in a syringe that solidifies into a durable embolus upon delivery without polymerization or dimethyl-sulfoxide (DMSO) precipitation.*
About Fluidx Medical Technology:
Fluidx Medical Technology is a Salt Lake City, Utah based company focused on developing the GPX Embolic Device and other embolic technologies with applications across peripheral vascular, interventional oncology, and neurovascular.
The GPX Embolic Device is under development and does not have marketing clearance or approval in any market at this time.
*Data on file. Fluidx Medical.