
Metastatic Tumor Treatment with Novel GPX Embolic Device
March 8, 2022
As part of an ongoing multi-center study, Fluidx Medical released additional information on treating tumors including a highly vascularized metastatic renal cell carcinoma with the GPX Embolic Device.
“We were very pleased with the outcome and the resulting occlusion created by GPX,” said Martin Krauss, M.D., Head of Interventional Radiology, Christchurch Hospital, Christchurch, New Zealand. “In one procedure, the renal cell carcinoma had metastasized to the upper femur/pelvic region. We used a combination of GPX and coils during the case. We treated five different tumor vessel segments with GPX.”
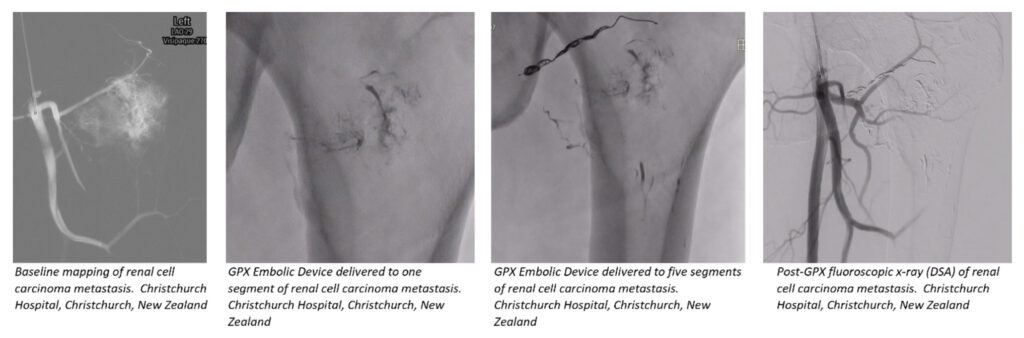
“GPX successfully occluded a variety of vessel presentations from smaller low flow vessels to larger higher flowing feeding vessels,” said Christopher Phillips, Director of Clinical Affairs. “GPX was delivered using a range of microcatheters and showed versatility in treating small and large metastatic tumor vessel beds. GPX was delivered through standard microcatheters that were appropriate for each targeted vessel’s size and blood flow.”
The GPX Embolic Device is an innovative embolic designed for simple preparation and controlled delivery. The device is packaged ready-to-use in a syringe, can be prepped tableside by the clinician in about 30 seconds, and may be delivered through standard microcatheters (no complex mixing systems or special delivery catheters are necessary).*
GPX technology is a low viscosity, aqueous-based solution in a syringe that solidifies into a durable embolus upon delivery without polymerization or dimethyl-sulfoxide (DMSO) precipitation. GPX is designed to occlude blood vessels independent of a patient’s coagulation situation.*
Fluidx Medical Technology is a Salt Lake City, Utah based company focused on developing the GPX Embolic Device and other innovative medical technologies.
The GPX Embolic Device is under development and does not have marketing clearance or approval in any market at this time. For investigational use (in New Zealand) only.
www.FluidxMedical.com
*Data on file. Fluidx Medical.